Capital and Concrete: This Week In Energy (June 30, 2025)
Google Bets on Fusion, GE Targets Nordic SMRs, Curve Eyes Low-Sulfur, US Picks Microreactors, Copenhagen Wins EIC
In The Headlines
Google Commits to First 200 MW of Fusion Power from CFS (CFS)
Google signs 200 MW PPA with Commonwealth Fusion Systems’ first ARC fusion plant in Virginia, targeting grid power by early 2030s
Deepens investment in CFS, reinforcing partnership and securing options to buy power from future ARC plants
Agreement hinges on SPARC tokamak achieving Q>1 net energy, enabling ARC to deliver 400 MW clean baseload power
An interesting question is why Google chose not to purchase the entire output of the reactor, given that its data centers consume massive amounts of energy. Another noteworthy connection is that Rick Needham, CFS’s Chief Commercial Officer, previously worked at Google—suggesting this deal may have been influenced by existing relationships.
Fusion’s limitless fuel potential positions it to meet surging AI-driven electricity demand and accelerate global energy transition
GE Vernova, Fortum Target Nordic SMRs (EN)
GE Vernova Hitachi Nuclear Energy and Fortum sign deal to prepare BWRX-300 SMR deployment in Finland and Sweden, aiming for 2030s commissioning
Fortum’s existing Nordic nuclear fleet includes Loviisa (10% of Finland’s power) and stakes in Olkiluoto, Forsmark, and Oskarshamn
BWRX-300 gaining global traction with projects like Ontario’s Darlington; cooperation lays groundwork for Scandinavian rollout
Curve Energy, Aramco Ink NDA on Low-Sulfur Marine Fuels (EN)
Curve Energy Corp. signs NDA with Saudi Aramco Technologies, enabling confidential talks on potential collaboration in low-sulfur marine fuels
Curve’s patented desulfurization tech converts HSFO to VLSFO at near-ambient conditions without SMR (Steam Methane Reforming) hydrogen, avoiding blends or scrubbers
Saudi Aramco eyes tech to boost environmental quality of hydrocarbon products; no details on project scope or timelines disclosed
US Picks Westinghouse, Radiant for First Microreactor Tests (EN)
DOE selects Westinghouse and Radiant Nuclear to launch first US microreactor trials at Idaho’s DOME facility, starting spring 2026
Westinghouse to test eVinci microreactor (5 MWe target) using heat pipe passive cooling for off-grid and industrial uses
Radiant’s Kaleidos unit aims to replace diesel gensets, generating 1.2 MWe in a containerized, deployable system
Copenhagen Atomics Wins EIC Funding for Thorium MSRs (Copenhagen Atomics)
Copenhagen Atomics awarded EUR 2.5mm (~$3mm) grant plus access to EUR 15mm (~$18mm) equity from EIC Accelerator, among only 40 startups selected
Funding supports development of containerized thorium molten salt reactors producing 100 MWth, able to recycle nuclear waste
Launchpad: Energy Startup Activity
Takanock Secures $500mm from ArcLight, DigitalBridge for Data Center Power (DigitalBridge)
Takanock raises $500mm from ArcLight and DigitalBridge to deploy on-site power solutions addressing data center energy constraints in Tier I markets
Founded in 2023 by Kenneth Davies (ex-Google Energy, Microsoft renewables), Takanock provides scalable, flexible prime power that transitions to grid support, reducing dependence on delayed utility generation and enhancing grid resiliency
Solutions accelerate time to power, avoid pipeline bottlenecks, and integrate best emission controls with closed-loop cooling to lower environmental impact while supporting renewable integration
Takanock’s natural gas-based systems deliver prime power on-site until substations come online, then shift to act as dispatchable grid resources, helping Tier I/II data center markets like Northern Virginia and Phoenix overcome energy constraints and grow sustainably
Climeworks Raises $162mm to Scale DAC, Eyes Market Leadership (AInvest)
Climeworks secures $162mm funding, year’s largest carbon removal deal, positioning it to lead a market projected at $1tn by 2050
Advances third-generation Direct Air Capture (DAC) technology with doubled energy efficiency, higher throughput, and longer filter life to drive down CO₂ capture costs
Third-gen DAC integrates improved sorbent materials and optimized process cycles, cutting energy use per ton of CO₂ captured while enabling larger, modular plant designs
Current plants capture 36,000 tons CO₂/year, expanding blended portfolio with nature-based solutions like afforestation and biochar, locking in 6mm tons of supply agreements
Backed by top investors (Microsoft, GIC, Partners Group) at $1bn+ valuation, Climeworks is set to dominate as carbon removal demand accelerates
Atawey Raises EUR 22mm (~$26mm) to Scale Hydrogen Mobility Across Europe (InnoEnergy)
Atawey secures EUR 22mm funding led by Starquest, ARMOR GROUP, and France 2030 to expand hydrogen refueling infrastructure in Europe
Company has 51 stations deployed, EUR 18mm (~$21mm) 2024 revenue (+113%), and targets profitability by end-2025, with EUR 30mm (~$35mm) in confirmed orders
Funds will accelerate rollout in Italy, Spain, Benelux, and deliver modular, regulation-ready stations for heavy-duty and industrial use
Backed by historical investors (InnoEnergy, Groupe IDEC, EIFFAGE) and aiming to strengthen production, services, and training for Europe’s hydrogen ecosystem
Desulfurization of Gas: Turning Sour Gas into Sweet, Clean Fuel
Natural gas and petroleum fuels often contain unwanted sulfur compounds that must be removed to make the fuel safe and clean. This process of removing sulfur is known as gas desulfurization. In simple terms, it is how we turn “sour” gas into “sweet” gas – sour gas meaning it contains sulfur (usually hydrogen sulfide), and sweet gas meaning it’s been purified of sulfur. In this article, we will explain what sour vs. sweet gas means, how gas is extracted and processed, and the technologies used to remove sulfur. We’ll focus on the standard hydrodesulfurization (HDS) process and then explore new and emerging methods, especially near-ambient chemical desulfurization techniques that promise to remove sulfur under mild conditions.
Sour Gas vs. Sweet Gas: What Are They?
When natural gas comes out of the ground, it doesn’t come as pure, clean methane. Raw natural gas from a well typically contains a mix of methane and other hydrocarbons, plus various contaminants: water, carbon dioxide, and often hydrogen sulfide (H₂S). If the gas contains significant H₂S, it’s called sour gas. If it has little to no H₂S, it’s called sweet gas. In other words, sour gas is “stinky” and corrosive due to sulfur, whereas sweet gas is the cleaner gas that is safe for pipelines and use.
How much H₂S makes gas “sour”? Definitions vary by location, but even a few parts per million of H₂S can qualify gas as sour. Some natural gas reservoirs have extremely high H₂S – in rare cases, gas can be almost half hydrogen sulfide or more. This H₂S is highly toxic (it smells like rotten eggs at low levels but can be deadly at high concentrations) and, when combined with water, it forms acids that corrode pipelines and equipment (a problem known as sulfide stress cracking). For these reasons, any gas with notable H₂S must be treated before it can be transported or used.
Extraction and Initial Processing of Natural Gas
Natural gas extraction involves drilling wells into underground reservoirs. Gas may come from gas-only wells or alongside crude oil (as “associated gas”). As it flows up, the pressure drops and liquids (like oil or condensate and water) may separate out. The raw gas that reaches the surface is often wet (mixed with liquids) and may be sour if H₂S or carbon dioxide (CO₂) are present. Before this gas can be sent through pipelines or burned, it must undergo processing to remove impurities.
In a typical natural gas processing plant, the gas is first dehydrated (to remove water) and separated from any liquid hydrocarbons. Then comes gas sweetening, which is the removal of acid gases like H₂S and CO₂. Commonly, this sweetening is done by bubbling the gas through a liquid chemical (an amine solution) that absorbs H₂S/CO₂. For example, amine treating uses solutions like monoethanolamine (MEA) to chemically capture H₂S. The now “sweet” gas that exits has very low sulfur and can meet pipeline standards. The H₂S is stripped from the amine solution and then converted into elemental sulfur or sulfuric acid as a by-product. In fact, removed H₂S is most often turned into solid sulfur in a unit called the Claus process. This way, a poisonous gas is transformed into useful sulfur, and the natural gas is rendered clean (sweet) for use.
Summary of sour gas processing: In short, raw extracted gas that is sour must be sweetened by removing H₂S and other sulfur compounds before use. This protects pipelines from corrosion and prevents releasing toxic sulfur gases when the fuel is burned.
Why Must Sulfur Be Removed?
Removing sulfur is critical for both safety/environment and equipment protection. As noted, H₂S in fuel gas is toxic and corrosive. Additionally, when any sulfur compounds in fuel are burned (whether in a power plant, car engine, or home furnace), they form sulfur dioxide (SO₂) gas. SO₂ is a pollutant that causes acid rain and respiratory problems. By desulfurizing gas and fuels, we greatly reduce SO₂ emissions and pollution. Modern environmental regulations require extremely low sulfur levels in fuels – for example, ultra-low-sulfur diesel has under 15 ppm sulfur – to minimize air pollution.
Sulfur also poisons certain catalysts. In oil refineries, even tiny sulfur amounts can damage expensive catalysts used in processes like catalytic reforming. Therefore, refineries remove sulfur early, to protect their processes. In summary, desulfurization is done to make fuels cleaner (for the air) and to prevent damage to infrastructure and catalysts.
Hydrodesulfurization (HDS): The Standard Desulfurization Process
The workhorse technology for removing sulfur from refinery streams (and sometimes from natural gas streams) is called hydrodesulfurization (HDS). Hydrodesulfurization is a catalytic chemical process that uses hydrogen gas to knock sulfur out of hydrocarbons. Essentially, the sulfur is reacted with hydrogen to form hydrogen sulfide (H₂S), which can then be separated out. The sulfur leaves the fuel and becomes H₂S gas, and the fuel molecule is left as a sulfur-free hydrocarbon. For example, a simple reaction in HDS would be: R-SH + H₂ → R-H + H₂S, where R-SH is a sulfur-containing molecule (like a thiol) and R-H is now a clean hydrocarbon. The H₂S produced is then removed and sent off to a sulfur recovery unit (Claus plant) to become elemental sulfur.
How HDS Works: In practice, HDS is carried out in large reactors packed with a solid catalyst (typically made of metal like cobalt or nickel combined with molybdenum on an alumina base). The fuel (e.g. diesel or gasoline blend stock, or other refinery stream) is mixed with high-pressure hydrogen and passed over the hot catalyst bed. The sulfur compounds in the fuel react on the catalyst surface with hydrogen, breaking the sulfur-carbon bonds. This produces H₂S gas and sulfur-free hydrocarbons. The clean fuel comes out with much lower sulfur content, and the H₂S by-product is captured downstream.
HDS units are found in almost every modern refinery because they are very effective at reducing sulfur to meet fuel standards. For instance, producing ultra-low-sulfur diesel for vehicles is achieved by HDS treatment. HDS is also applied (on a smaller scale) in processing natural gas liquids or to treat natural gas if it contains certain organic sulfur molecules. Thanks to HDS and similar processes, the majority of sulfur in crude oil and gas ultimately ends up as recovered elemental sulfur. In fact, most of the world’s elemental sulfur supply is a byproduct of hydrocarbon processing (recovered from H₂S gas generated by HDS and gas sweetening).
The Downside of HDS: Hydrodesulfurization is effective but also has significant challenges. The process requires rather harsh operating conditions: typical HDS reactors run at hundreds of degrees Celsius and high pressures of hydrogen gas. For example, treating heavy oil or bunker fuel might require around 400–500 °C and 50–120 atmospheres of pressure of H₂. These extreme conditions mean HDS units consume a lot of energy. They also consume a lot of hydrogen gas – which is costly and usually made from natural gas with a sizable carbon footprint (manufacturing 1 ton of hydrogen can emit ~14 tons of CO₂). Furthermore, the catalysts used in HDS (usually based on molybdenum with cobalt/nickel) can be poisoned by other impurities and eventually wear out. Spent HDS catalysts are considered hazardous waste and need proper disposal. Another issue is that certain sulfur compounds, especially larger, ring-shaped molecules like dibenzothiophenes in heavy oil, are very refractory (hard to desulfurize). HDS struggles to remove these completely unless the process conditions are made even more severe (higher temperature, more hydrogen). Pushing HDS to those extremes raises costs and can make the process uneconomical for smaller refineries. To summarize the challenges of conventional HDS:
High Severity Requirements: Needs high temperature and pressure hydrogen, making the process energy-intensive and expensive
Hydrogen Consumption: Uses large amounts of hydrogen gas, which is costly and comes with a CO₂ emissions penalty to produce
Catalyst Issues: Catalysts can be poisoned by impurities and produce waste when spent
Incomplete for Hard Sulfur Compounds: Certain complex sulfur molecules cannot be fully removed without extremely severe conditions
These limitations drive up the cost of desulfurization and have motivated researchers to seek alternative methods that can operate under milder conditions.
Emerging Methods for Desulfurization at Near-Ambient Conditions
Given the drawbacks of HDS, there is great interest in new desulfurization technologies that require less extreme conditions (ideally near-ambient temperature and pressure) and do not rely on consuming hydrogen. Several approaches have been explored and are in various stages of development. We will discuss a few notable ones: oxidative desulfurization, solvent-based extraction methods, adsorptive desulfurization, and biodesulfurization. These methods aim to remove sulfur either by chemically transforming sulfur compounds in mild conditions or by capturing them without the need for high-pressure hydrogen. Many of these can be performed at or near ambient temperatures, making them attractive complements or alternatives to HDS.
Oxidative Desulfurization (ODS)
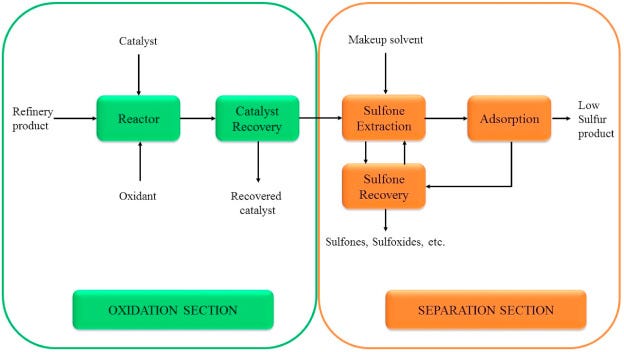
Oxidative desulfurization (ODS) is a chemical approach that, instead of using hydrogen to remove sulfur, uses an oxidizing agent to transform sulfur compounds into a form that can be easily separated. The idea is that most sulfur in fuels is in forms like sulfides, thiophenes, etc., which are relatively non-polar. If you oxidize them, they turn into sulfoxides or sulfones – these oxidized sulfur compounds are more polar or higher-boiling, and thus can be extracted out or filtered out of the fuel. ODS typically operates at much lower temperatures than HDS, often near ambient temperature and atmospheric pressure, because strong chemical oxidants are doing the work instead of heat and hydrogen.
In an ODS process, one might mix the fuel with an oxidant (such as hydrogen peroxide, organic peracids, or even air in presence of a catalyst) and a catalyst to speed up the reaction. The oxidant attacks the sulfur compound, for example converting a thioether (R-S-R) into a sulfone (R-SO₂-R). An important feature is that this can be done on relatively mild conditions. For instance, researchers have reported that with the help of catalysts like ferric chloride (Fe³⁺), thiophenic sulfur compounds in fuel could be oxidized to sulfoxides/sulfones at room temperature (~25 °C). This is a dramatic difference from the 300+ °C needed in HDS. Once oxidized, those sulfur compounds (now sulfones) are far easier to remove. They may either precipitate out, or more often, they can be washed out by extraction with a polar solvent or removed by adsorption.
One example of an ODS-based process is the Conversion and Extraction Desulfurization (CED) process developed by Petro Star and partners. In this process, sulfur compounds in diesel fuel were selectively oxidized and then extracted, all under near-ambient conditions without using any hydrogen gas. Because it doesn’t need high heat or pressure, CED avoided the costly hydrogen-based HDS route. In trials, the CED process could reduce diesel sulfur from 0.5% (5000 ppm) down to below 0.001% (10 ppm) in the lab. This demonstrates that ODS paired with extraction can achieve ultra-low sulfur levels. However, the pilot plant trials showed a bit of difficulty reaching the final 15 ppm target consistently (they got ~20 ppm), highlighting that while promising, ODS processes still may need optimization to match the absolute performance of HDS. Even so, the appeal of ODS is clear – no hydrogen, no extreme reactors, and it can even tackle some sulfur molecules that HDS has trouble with (since strong oxidants don’t mind aromatic rings as much).
After oxidation, a separation step is needed. Often a water-based or alcohol-based solvent is used to wash out the new sulfone/sulfoxide compounds. Because those oxidized sulfur compounds are more polar, they preferentially move into the polar solvent phase, leaving the clean hydrocarbons in the oil phase. This oxidation + extraction combination is a common theme in many ODS methods. In fact, oxidizing the sulfur greatly improves how well solvents can extract them – essentially, you make the sulfur compounds easier to pull out. The net result is a sweetened fuel without using hydrogen. One must manage the used oxidants and any by-products (for example, sulfones might be collected and potentially processed or discarded), but those are generally easier to handle than large volumes of H₂S gas.
ODS is a very active area of research. Various oxidants (like peracids, ozone, even oxidation using catalysts with air/O₂) and various catalysts (to make oxidation faster and selective) have been studied. Some processes use ultrasound or microwaves to accelerate the oxidation at low temps (ultrasound creates cavitation that can boost the reaction). Others integrate ionic liquids as the extraction solvent after oxidation. The key advantage is always the same: operate at low temperature and ambient pressure, and avoid consuming hydrogen. The challenge ODS faces is handling the oxidant cost and the disposal of oxidized sulfur byproducts. Unlike HDS, which converts sulfur to H₂S gas that can be easily processed to elemental sulfur, ODS produces liquid sulfur compounds that you have to separate and deal with. If those can be economically handled (for example, some processes might recover the sulfones into an asphalt additive or some use), ODS could be a game-changer for small refineries or even for treating fuels like marine fuel oil on-site.
Solvent-Based Extraction (Physical Sweetening)
Another approach to desulfurization is to skip chemical reactions and use extractive desulfurization, which is essentially a physical process. This means using a solvent that preferentially dissolves sulfur compounds out of the fuel. You mix the fuel with an immiscible solvent, let it pick up the sulfur components, then separate the solvent and recover the fuel with lower sulfur. This method has been experimented with various solvents, including organic solvents and special ones like ionic liquids.
The appeal of extraction is its simplicity: it can be done at moderate or near-ambient conditions since it’s just mixing and settling phases – no high-pressure reactors needed. However, it’s not easy to find the perfect solvent. The solvent must strongly prefer sulfur compounds over the bulk hydrocarbons (to pull enough sulfur out), yet not mix too much with the hydrocarbon phase itself. Solvents like methanol, acetone, or polyethylene glycol have been tested and can remove 50–90% of sulfur in repeated extractions. Ionic liquids (salts that are liquid at room temperature) have also been studied because they don’t evaporate and can be engineered to have an affinity for sulfur compounds. Some imidazolium-based ionic liquids, for example, show good pickup of thiophenic sulfur. But in real fuels, extraction alone often falls short of ultra-deep desulfurization because many sulfur molecules are still more soluble in the oil than in the solvent, especially the nasty polyaromatic sulfur heterocycles.
One way to improve extraction is – as noted above – to oxidize first then extract. Oxidation makes the sulfur molecules more polar, which dramatically improves the distribution into a polar solvent. So, extraction is frequently discussed hand-in-hand with ODS (oxidative + extractive desulfurization combined). Without oxidation, pure extraction might reduce sulfur moderately but usually can’t reach the ultra-low levels required. Moreover, handling large volumes of solvent and energy needed to recover and recycle the solvent can be an issue. Thus, while solvent extraction is conceptually simple and mild (and indeed, it’s essentially what we do in labs to analyze sulfur compounds), its practical use in large-scale fuel desulfurization is limited unless paired with other tricks.
Adsorptive Desulfurization
Adsorptive desulfurization involves using solid materials (sorbents) that can adsorb sulfur-containing molecules from fuels or gas. In this method, the fuel is passed through a bed of sorbent (like a filter), and the sorbent captures sulfur compounds on its surface, letting clean fuel out. Some sorbents can bind sulfur very selectively. For instance, metal-loaded zeolites or metal-organic frameworks have been researched for this purpose – certain metals (like copper or silver) have a chemical affinity for sulfur atoms and can grab sulfur compounds out of the mixture. The big advantage here is that adsorption can be done at ambient temperature and pressure as well, and it doesn’t require adding other chemicals. It’s akin to having a sulfur sponge.
One notable example was a commercial process named “S-Zorb” (by ConocoPhillips) which used a solid sorbent to remove sulfur from gasoline. However, S-Zorb still needed fairly high temperature to make the sulfur react with the sorbent. Research continues into room-temperature adsorbents for fuels. In fuel cell applications, for example, where sulfur has to be virtually zero to avoid poisoning the fuel cell catalyst, people have looked at on-board ambient-temperature adsorbent filters to polish fuel. Studies have shown that using tailored zeolites at near-ambient conditions can indeed reduce sulfur dramatically. The trick is the sorbent capacity – once it’s saturated with sulfur, you either have to regenerate it (heat it to release or destroy the sulfur compounds) or replace it. For natural gas streams, a very common adsorptive approach is using zinc oxide beds to remove the last traces of H₂S. ZnO reacts with H₂S to form solid zinc sulfide and water – this is a chemisorption process and is done at relatively warm temperatures (a few hundred °C usually), but it’s another example of adsorption used industrially (especially to protect downstream catalysts from sulfur). Newer research sorbents aim to work at lower temperatures and be regenerable.
In summary, adsorptive desulfurization can be thought of as filtering out sulfur compounds using a special filter material. It’s potentially very useful for final clean-up of fuels to get that last few ppm of sulfur that other processes might miss. The challenge is maintaining efficiency and regenerating the sorbent cost-effectively.
Biodesulfurization (BDS)
A rather different approach is to use biology – certain microbes – to perform desulfurization. Biodesulfurization (BDS) leverages bacteria that can metabolize sulfur compounds. Some sulfur-eating bacteria can take an organosulfur molecule and essentially “consume” the sulfur (converting it to sulfate or hydrogen sulfide) while leaving the hydrocarbon part mostly intact. The classic example is using bacteria like Rhodococcus that can remove sulfur from dibenzothiophene via the 4S pathway, producing 2-hydroxybiphenyl (which is sulfur-free). The appeal of BDS is that it can operate at mild conditions – basically near ambient temperature and pressure, since these are living organisms, often doing their job in water. It’s a “green” solution in concept.
However, BDS has not yet seen commercial success for large-scale fuel desulfurization. The reasons include: it’s relatively slow (microbes take time to grow and react, whereas chemical processes are faster), the bacteria can be inhibited by other components in fuel (organic solvents like hydrocarbons are not a friendly environment for living things in high concentration), and separating the microbes or their enzymes from the oil phase can be tricky. There’s ongoing research into genetically engineering more robust bacteria or using enzymes in immobilized form to overcome these issues. In specialized cases, BDS might still find a niche – for example, treating some specific sulfur compound that is hard for HDS but easy for a certain enzyme, or in processing oils where HDS conditions would be too harsh (if mild processing is needed). For now, BDS remains a promising but challenging alternative.
The Future: Towards Cleaner Fuels with Less Energy
Desulfurization of gas and fuels will continue to be a crucial step in energy production as the world demands cleaner fuels and stricter environmental standards. Today, hydrodesulfurization (HDS) remains the dominant technology to produce sweet natural gas and low-sulfur gasoline, diesel, and other fuels. It is highly effective but comes with high energy and hydrogen costs. That’s why there is a strong push for near-ambient desulfurization technologies – processes that can achieve the same sulfur removal at lower temperature, lower pressure, and with less added hydrogen or chemicals.
Some of the emerging methods we discussed, like oxidative desulfurization coupled with extraction, show that it’s possible to attain ultra-low sulfur levels at mild conditions. Innovative solutions (for example, the proprietary process by Curve Energy for ship fuels) are being developed to upgrade high-sulfur oils to low-sulfur without the big carbon footprint of HDS, by using novel chemistry under near-ambient conditions. Adsorbent-based polishing units might complement larger processes to squeeze out the last bit of sulfur cheaply at the end of a treatment train. Biological methods, while not yet practical at scale, could one day play a role if engineered microbes become more efficient.
In practical terms, the industry might not replace HDS entirely anytime soon – it’s a well-established, continuous process that refineries are built around. But we may see hybrid approaches: for example, using an oxidative/adsorptive process to assist HDS, thereby lowering the severity needed in the HDS unit (saving hydrogen and energy), or to help small facilities that can’t afford huge HDS units to still meet sulfur specs. The drive for lower costs and lower environmental impact (including lower CO₂ emissions) will likely make these alternatives more attractive. Every barrel of oil or cubic meter of natural gas processed with a gentler desulfurization method means energy saved and potentially less CO₂ emitted.
Conclusion: Desulfurization of gas – taking “sour” gas and making it “sweet” – is essential for safe use of fossil fuels. It protects us from poisonous gases and prevents acid rain and pollution. The conventional methods like amine gas sweetening and hydrodesulfurization have done the job for decades, turning sulfur-rich resources into clean-burning fuel. Now, with modern demands, the focus is on improving this process. Near-ambient chemical desulfurization methods represent the cutting edge of this field, promising sulfur removal with less heat and pressure. As these technologies mature, we can look forward to cleaner fuels produced with greener processes, continuing the effort to reduce the environmental footprint of the energy we use every day.